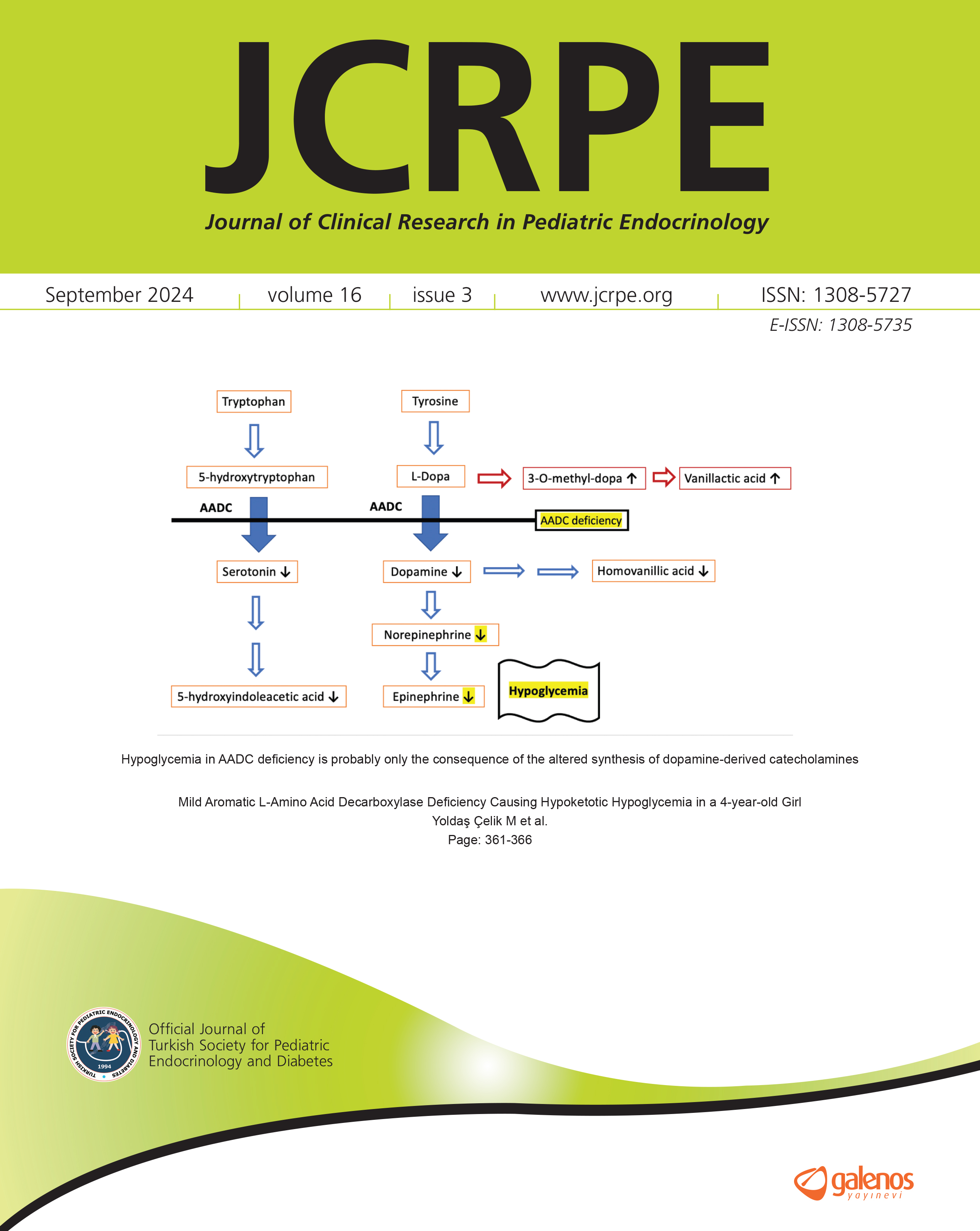
Liraglutide Treatment Improves Glycaemic Dysregulation, Body Composition, Cardiometabolic Variables and Uncontrolled Eating Behaviour in Adolescents with Severe Obesity
L. Apperley, J. Parkinson, S. SenniappanDepartment of Paediatric Endocrinology, Alder Hey Childrens Hospital, Liverpool, UKINTRODUCTION: Childhood obesity is associated with long-term health complications. Liraglutide is approved for use in adolescents for weight loss and has shown beneficial outcomes in clinical trials. Continuous glucose monitoring (CGM) is routinely used in type 1 diabetes mellitus. We aimed to look at the effect of liraglutide treatment on cardiometabolic variables, glycaemic control (as assessed by CGM), body composition, quality-of-life and satiety levels in adolescents with severe obesity.
METHODS: 24 patients aged 12 to 17.9 years (10M: 14F) were commenced on liraglutide in addition to lifestyle support. PedsQL 4.0 generic scale and Three-factor Eating Questionnaire R18 were completed at baseline and 3-months.
RESULTS: Significant improvements were shown in weight, body mass index, body mass index standard deviation scores, percentage body fat and fat mass following liraglutide treatment. A significant reduction in HbA1c, triglyceride and cholesterol levels, as well as a reduction in uncontrolled eating behaviour were observed. When compared to the healthy adolescents, the time spent within normal glucose range (3.9-7.8mmol/L; 70.2-140.4 mg/dL) remained low (91.76% vs 97.00%) at baseline but improved after liraglutide treatment. Our results showed lower health-related quality-of-life scores and higher uncontrolled eating and emotional eating behaviours, compared to the healthy population.
DISCUSSION AND CONCLUSION: We report, for the first time, the role of CGM in identifying glycaemic dysregulation in children and young people with obesity before and after liraglutide treatment. The results have shown significant potential for liraglutide treatment in improving the outcomes. Earlier identification of glycaemic dysregulation and targeted therapy could potentially reduce the long-term risk of developing T2DM.
Manuscript Language: English